Yoguely is reader-supported. When you buy through links on our site, we may earn an affiliate commission. Learn more
How to Use Hydrogen Peroxide Disinfectant (0.5% vs 3% vs 35%)

In today’s post I’m going to show you exactly how to use hydrogen peroxide the right way to safely disinfect surfaces from 99.99% of microbes including COVID-19.
In fact, this is the same process I use to choose the best ratio with water and safely disinfect using hydrogen peroxide.
So if you would like to know “How to clean with hydrogen peroxide?”, you’ll love this guide.
Let’s jump into it!
How to Use It the Right Way
Hydrogen peroxide is a staple cleaning agent and the active ingredient in many household cleaning solutions.
It is nice to use because you do not need to dilute it yourself. It comes diluted with purified water or with other inactive ingredients to a variety of concentration levels.
The hydrogen peroxide (H2O2) (paid link) is typically diluted to 3% for topical solutions. It can also be found at concentrations between 0.4% to 8%, and even up to 35% for solutions marketed as cleaning agents.

I’ll go over which one is the best very soon.
Similar to sodium hypochlorite (the common bleach), hydrogen peroxide is also a bleaching agent.[1] As a bleach, it reacts with organic compounds and turns them colorless.[30]
Hydrogen peroxide is perceived as an environmentally safe alternative to sodium hypochlorite because it degrades into oxygen and water, which isn’t harmful to the environment.[1]
2 H2O2 → 2 H2O + O2
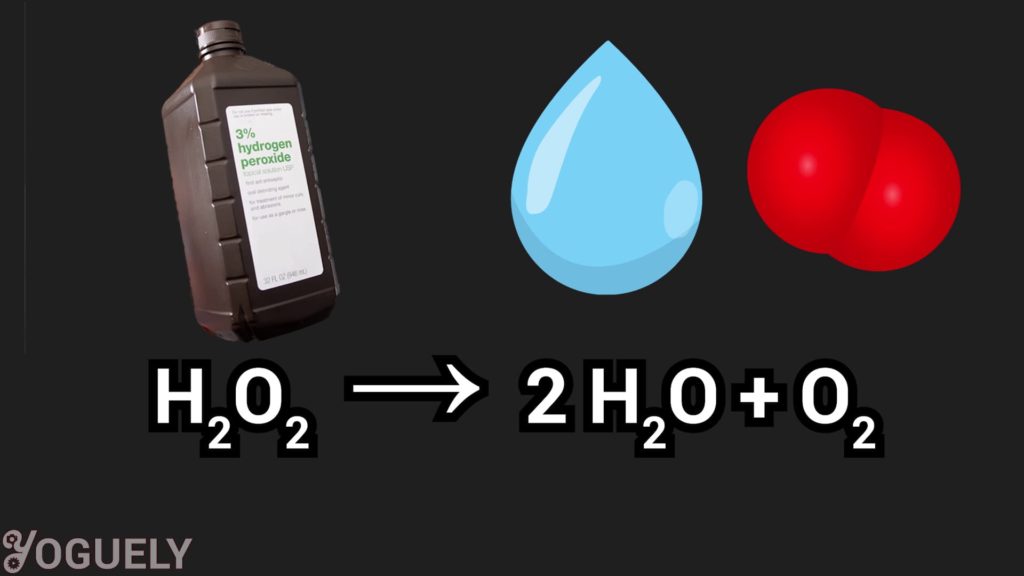
Because it is a bleaching agent, it could also serve as a disinfectant. I’ll go over how effective of a disinfectant it is very soon.
First know that you’ll first want to clean the surface with detergent or soap and water to remove as much dirt as possible before using hydrogen peroxide.
What is Hydrogen Peroxide Good For?
Material it helps remove: Hydrogen peroxide is a helpful cleaning agent because it breaks up debris, protein, and fatty acids.[4]
Since it is a bleach, it is also very effective in removing stains. I personally use is when I get pasta sauce on my shirt or I’ve spilled blackberry smoothie on the carpet.
Surfaces you can clean: Use hydrogen peroxide on porous and even on non-porous materials.[10] So feel safe to use it to clean marble and granite counter tops in your kitchen.

If it gets trapped in the pores, it will naturally break down to water and oxygen with time. Or you could just rinse it off.
Is Hydrogen Peroxide a Disinfectant?
According to the CDC and the EPA, hydrogen peroxide is considered a disinfectant.[3],[32]
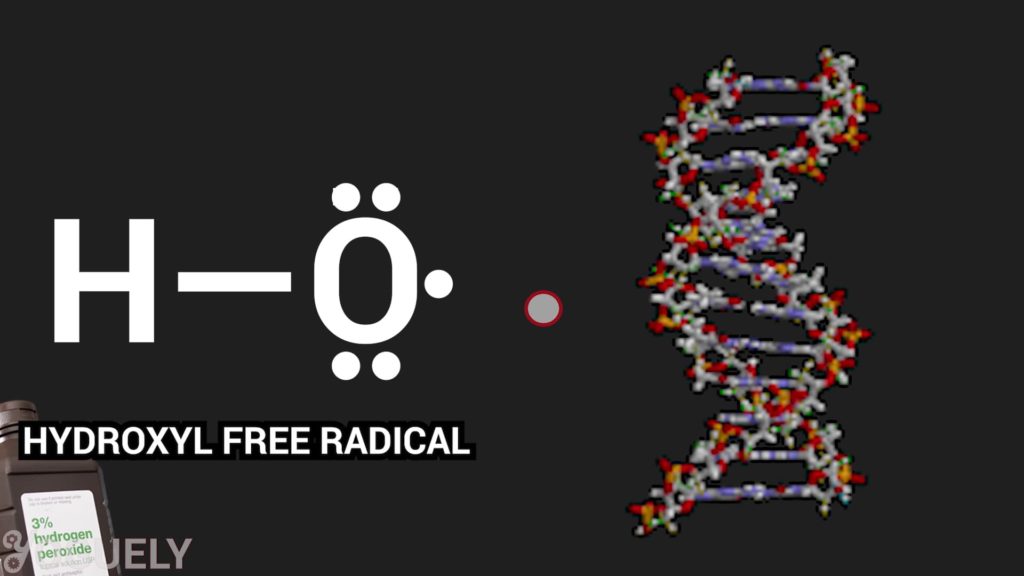
It works as a disinfectant by producing hydroxyl free radicals (•OH).[3]
Free radicals are atoms, molecules, or ions that have an unpaired valence electron.[5] Because free radicals are missing an electron, they really want to steal an electron and fill that void, which makes them highly reactive.
These hydroxyl radicals can attack and destroy membrane lipids, DNA, and other essential cell components. Which means that hydrogen peroxide essentially disinfects by destroying or inactivating microbes.
How effective is hydrogen peroxide as a disinfectant? Hydrogen peroxide is very effective as a disinfectant against microorganisms like yeasts, fungi, bacteria, bacterial spores, and viruses.[3]
However, some organisms have a defense mechanism against H2O2.
How do some organisms protect themselves from hydrogen peroxide?
Some organisms produce a protein called catalase that serves as way to protect against annihilation.
This protein catalase is an enzyme that accelerates a really neat chemical reaction. The degradation of hydrogen peroxide into harmless water and oxygen.[3]
2 H2O2 → 2 H2O + O2
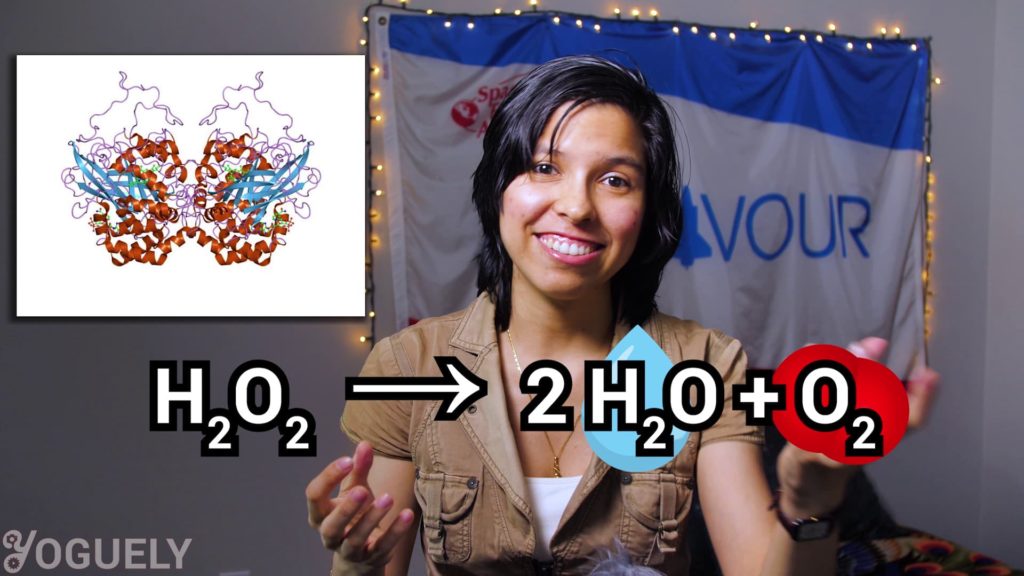
So,
What can you do to these organisms that can tolerate a bath of hydrogen peroxide?
Use longer contact times and higher concentrations and their defense mechanism won’t be able to hold off.[6],[7]
What Concentration of Hydrogen Peroxide Do I Need to Disinfect?
In other words, what is the best hydrogen peroxide disinfectant ratio with water?
According to the CDC, the most common commercially available 3% hydrogen peroxide is a stable and effective disinfectant when used on inanimate surfaces.[33]
Which implies that it is a cleaning agent that is effective in killing 99.99% of germs.
Which raises a critical question.
What about the 0.01% of germs it doesn’t destroy?
Well you’ll need to make sure that whatever germ you want to get rid of isn’t in the 0.01%.
According to the CDC, the concentration you need will depend on the microorganism you wish to eradicate and how long of a contact time you would like to achieve.[3] The general trend is: the higher the concentration, the lower the contact time needed.
For a 3% solution, it would take up to 10 minutes to destroy or inactivate viruses, and up to 150 minutes to kill spores.
| Microorganism | Contact Time (min) |
|---|---|
| Three serotypes of rhinovirus (predominant cause of the common cold)[3] | 6 to 8 |
| Coronavirus (COVID-19)[32] | 1 to 10 |
| Spores (Bacillus species)[3] | 150 |
So instead of keeping a high concentration solution in your residence, consider only storing lower concentrations, and just increasing the contact time when you need.
It will be safer, and you can use it for other applications aside from disinfecting surfaces. I’ll dive more in to that real soon.
Can Hydrogen Peroxide Kill Coronavirus?
Yes, hydrogen peroxide is an effective disinfectant against the novel coronavirus. The Environmental Protection Agency (EPA) registers it as an effective active ingredient to disinfect SARS-CoV-2 (COVID-19).[32]
How Long Should Hydrogen Peroxide Sit on Surface to Disinfect?
According to the EPA, the amount of time the surface should be visibly wet with the disinfectant is called the contact time.[32]
Contact time: The surface you wish to disinfect will need enough contact time with hydrogen peroxide to kill the majority of germs.
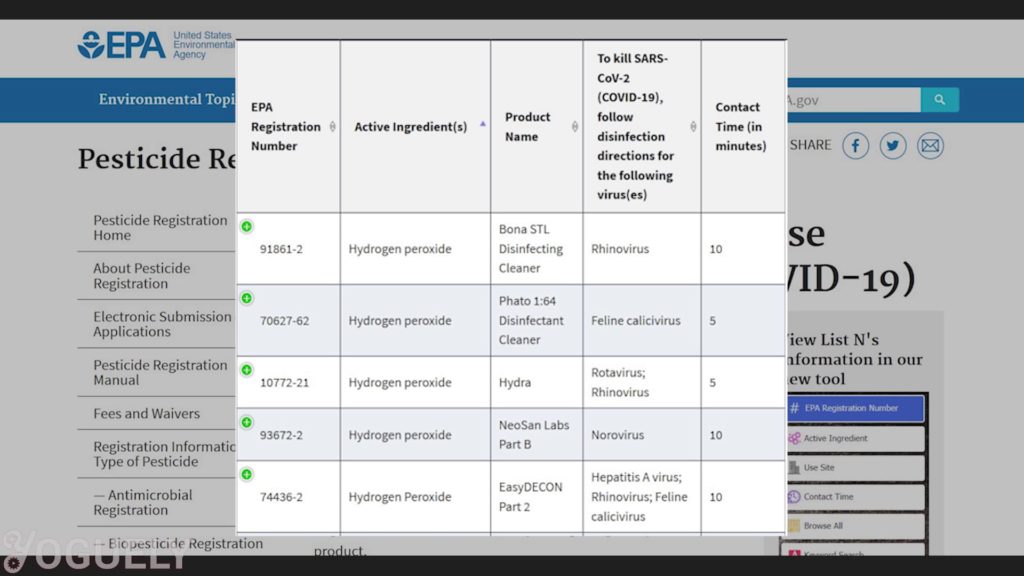
Therefore, the contact time depends on the microbe you wish to destroy and the concentration used. For more info, check out the lists of EPA-Registered Disinfectants for common pathogens.[34]
I’ll be focusing on COVID-19, since it is one of the biggest threats to our well-being we face today.
The EPA recommends as much as 10 minutes of contact time with solutions of 3% sodium hypochlorite to be effective against COVID-19.[32]
I looked into the MSDS sheet for each EPA-Registered product containing hydrogen peroxide as its sole active ingredient. With what I found, I plotted how percent solution concentration trends with contact time needed for COVID-19.
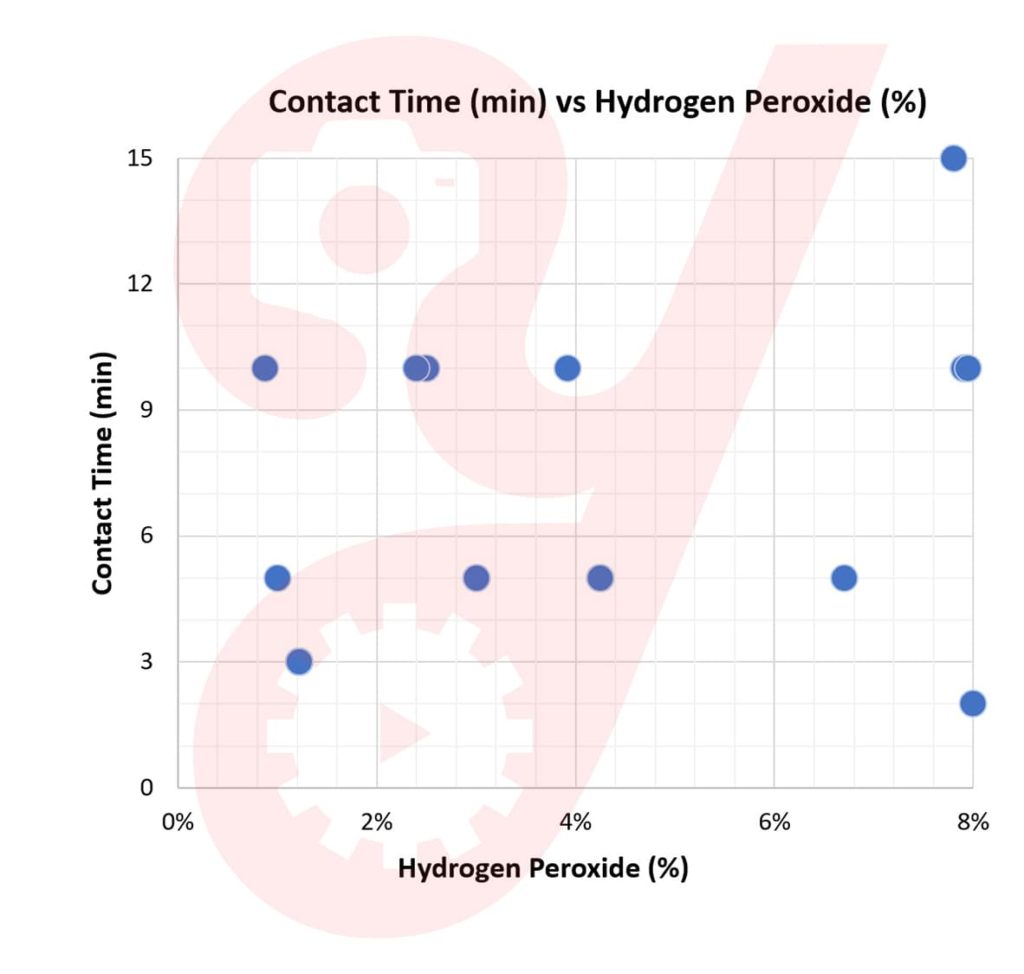
The data is scattered, with a minimum contact time of 2 minutes and a maximum of 15 minutes for concentrations of up to 8%.
35% Hydrogen Peroxide
There are products containing 35% concentration of hydrogen peroxide which are extremely dangerous to handle at home. They are toxic pesticides to life and the environment.
It is so harmful if accidentally ingested that in 2006, the FDA tried to warm consumers not to purchase high-strength 35% hydrogen peroxide for medicinal use.[9]
The EPA’s website doesn’t even list a contact time for products with 35% hydrogen peroxide, it just says to consult the user manual.[32]
In a household setting, you will probably never need to use such high concentrations since it may bring more harm than good.
0.5% Accelerated Hydrogen Peroxide® (AHP®)
There is a special kind of product, and a generally more expensive product, containing 0.5% hydrogen peroxide with specific additives.
It is patented as Accelerated Hydrogen Peroxide® (AHP®). Since this one behaves very differently from regular hydrogen peroxide, I did not include it in my plot above.
Products with AHP® will need only 30 seconds or up to 1 minute of contact time to be effective against coronavirus (COVID-19).[59]-[62]
That’s really fast, huh?
I was very impressed when I first saw the marketing, so let me tell you more about it so you make a wise choice next time you are shopping.
What Is the Difference Between Hydrogen Peroxide and Accelerated Hydrogen Peroxide® (AHP®)?
Common hydrogen peroxide is H2O2 diluted in water.
Accelerated hydrogen peroxide® (AHP®) has the same active ingredient hydrogen peroxide with a proprietary formula of non-active ingredients consisting of surfactants and organic acids.[8]
The formula is proprietary so who knows what is in it. What we do know is that AHP typically uses a low level of hydrogen peroxide, only 0.5%.
The difference in disinfection is that AHP® disinfects much quicker. The contact time for AHP® is between 30 seconds and a minute.[32],[33]
The idea is that AHP® “speeds up” the time it takes to disinfect a surface.
For this reason, AHP® is being marketed as safer, since you don’t have to leave it on surfaces for so long.
There is also a BIG difference in price. For instance, this RTU disinfectant with AHP® (paid link) is about 16 times more expensive than regular 3% hydrogen peroxide
Which is better Hydrogen Peroxide or Accelerated Hydrogen Peroxide® (AHP®)?
So here is my opinion on AHP® vs regular hydrogen peroxide.
Regular 3% hydrogen peroxide is already pretty safe to use (with care of course), and you can use it for a whole lot of things other than disinfecting surfaces. You can also use it to disinfect wounds, and create your own laundry detergent. More on that soon.
So that is 3-purposes in 1! A multipurpose solution!
Consider a systems approach to this problem of disinfecting at home. The best solutions are those that solves many problems while not creating a new one.
The key advantage to AHP® is reducing contact time. But I’ve got time, so that is not a problem for me at the moment.
Instead AHP® create three new problems:
- The added cost means that I will have to work longer in my life time or become more productive to be able to cover those costs.
- It is not a topical solution for cuts and abrasions on the skin. So instead of owning one product, I would need two different products which occupy more space at home.
- The lack of publicly available scientific research on the long-term effects of the particular mix of chemicals creates uncertainty of the degree of harm to our health and the environment.
Other Uses for Hydrogen Peroxide
First aid antiseptic
Hydrogen peroxide that contains just 3% concentration diluted only with purified water can be used as a first aid antiseptic.
It is marketed most commonly as a topical solution. That is, a solution that you can apply to a particular part of your body.

It can help prevent disease-causing microorganisms from growing on your wounds. Because of this, people use it as a oral debriding agent, to disinfect or help remove damaged tissues from oral wounds.
However, it has been found to interfere with wound healing in a way that induces scars.[2] So you may be better off using isopropyl alcohol to disinfect wounds.
Detergent
Hydrogen peroxide actually has more sanitation uses in the home. You can use it to make laundry detergent!
What you need to do is create sodium perborate. Sodium perborate is a mild bleach used in many popular laundry detergents.[1]
React borax (Na2B4O7 + 2 NaOH) with hydrogen peroxide (4 H2O2) and you’ll get sodium perborate (2 Na2B2O4(OH)4 ) and a water molecule.
Na2B4O7 + 2 NaOH + 4 H2O2 → 2 Na2B2O4(OH)4 + H2O
Now you know how to create laundry detergent! Cool huh?
Alrighty! Next let’s talk about it’s hazards and safety measures you should take.
Hydrogen Peroxide Safety Measures
Here are some safety measure to take whenever working with hydrogen peroxide.
I’m going to go over the most commonly sold solution at 3% concentration.
MSDS: Safety Data Sheet for Hydrogen Peroxide at 3% concentration.
Hazards: As far as hazards go, hydrogen peroxide is a strong oxidizer. So it may cause a fire or explosion. Furthermore it is corrosive, so it will cause severe skin burns and eye damage. Likewise, it is an irritant and is harmful if inhaled.

Personal Protective Equipment (PPE): When handling hydrogen peroxide, you should cover your skin with protective clothing, wear gloves (paid link), and safety goggles (paid link) or a full face shield.

Keep it away from heat and ignition sources to prevent triggering a fire.
In the event of a spill, you’ll want to have an emergency eye wash and safety shower nearby.
Also, ensure you have adequate ventilation.
Ok! After you are done working with it, you might be wondering,
What is the Shelf Life of Hydrogen Peroxide?
Shelf Life: A 3% hydrogen peroxide product will have a EXP date on printed on it which is about 3 years from the date you purchase it. But that might not mean that the product is completely unusable.
According to the CDC, if stored at ambient temperature in a dark container, the rate of loss of potency of hydrogen peroxide is less than 2% per year.[3]
Based on that, as the years pass, you would need to adjust the contact time to reach the same potency.
How to Store Hydrogen Peroxide for Long Term?
Hydrogen peroxide slowly decomposes in light. So keep it in an opaque container hidden away from light.[1]
Store the tightly sealed container in a cool location with ventilation.
Store it separately from other chemicals that could react adversely.
Remember to never mix hydrogen peroxide with vinegar since it forms peracetic acid which is highly corrosive.

Conclusion
There you have it folks. That’s my guide on how to use hydrogen peroxide the right way.
Leave your thoughts in the comment section below. Or join the discussion in the Yoguely Community Forum.
I’m Aida Yoguely. Thanks for learning with me today. And I’ll see you soon.
In my next post, learn about sodium hypochlorite, the active ingredient in bleach, and how to use it for disinfecting. To stay tuned, join our newsletter and get the latest content straight to your inbox.
Video
Be sure to subscribe and hit the notification bell to stay tuned for the latest videos.
References
1. ^ (May 26, 2020). “Hydrogen peroxide”. Wikipedia. Retrieved May 30, 2020.
2. ^ Wilgus, T. A., Bergdall, V. K., Dipietro, L. A., & Oberyszyn, T. M. (2005). Hydrogen peroxide disrupts scarless fetal wound repair. Wound Repair and Regeneration, 13(5), 513-519.
3. ^ (Sept 18, 2018). “Guideline for Disinfection and Sterilization in Healthcare Facilities (2008)”. CDC. Retrieved May 30, 2020.
4. ^ (Aug 30, 2018). “Hydrogen peroxide Solution”. FDA. Retrieved May 30, 2020.
5. ^ (May 17, 2018). “Radical (chemistry)”. Wikipedia. Retrieved May 30, 2020.
6. ^ Turner FJ. Hydrogen peroxide and other oxidant disinfectants. In: Block SS, ed. Disinfection, sterilization, and preservation. Philadelphia: Lea & Febiger, 1983:240-50.
7. ^ Block SS. Peroxygen compounds. In: Block SS, ed. Disinfection, sterilization, and preservation. Philadelphia: Lippincott Williams & Wilkins, 2001:185-204.
8. ^ (May 29, 2020). “Disinfectant”. Wikipedia. Retrieved May 31, 2020.
9. ^ (July 30, 2006). “FDA Warns Consumers Against Drinking High-Strength Hydrogen Peroxide For Medicinal Use”. Science Daily. Retrieved May 31, 2020.
10. ^ “Bleach Free Whitening Formula”. Fiberlock Technologies. Retrieved May 31, 2020.
11. ^ “Compound Summary: Hydrogen Peroxide”. National Library of Medicine. Retrieved June 1, 2020.
30. ^ (May 16, 2020). “Bleach”. Wikipedia. Retrieved May 26, 2020.
32. ^ “List N: Disinfectants for Use Against SARS-CoV-2”. EPA. Retrieved May 27, 2020.
33. ^ (2013). “Green Cleaning, Santizing, and Disinfecting: A Curriculum for Early Care and Education”. EPA. Retrieved May 27, 2020.
34. ^ (March 13, 2013). “Selected EPA-Registered Disinfectants”. EPA. Retrieved May 27, 2020.
40. ^ (June 2, 2016) “Hydra”. EPA. Retrieved May 31, 2020.
41. ^ (Dec 17, 2004) “EasyDECON Part 2”. EPA. Retrieved May 31, 2020.
42. ^ (Dec 3, 2018) “DS-6640”. EPA. Retrieved May 31, 2020.
43. ^ (Dec 3, 2018) “DS6809”. EPA. Retrieved May 31, 2020.
44. ^ (Nov 27, 2018) “Angel”. EPA. Retrieved May 31, 2020.
45. ^ (May 7, 2018) “B-Cap™ 35 Antimicrobial Agentfor Use Against SARS-CoV-2”. EPA. Retrieved May 31, 2020.
46. ^ (Dec 3, 2018) “Angel”. EPA. Retrieved May 31, 2020.
47. ^ (May 7, 2018) “D7 Part 2”. EPA. Retrieved May 31, 2020.
48. ^ “Vaprox® Hydrogen Peroxide Sterilant”. Steris life sciences. Retrieved May 31, 2020.
49. ^ (Dec 18, 2018) “Proxi Home General Disinfectant Cleaner Spray”. EPA. Retrieved May 31, 2020.
50. ^ (Dec 14, 2017) “HP2O2”. EPA. Retrieved May 31, 2020.
51. ^ (Sept 5, 2008) “Peroxy HDOX”. EPA. Retrieved May 31, 2020.
52. ^ (July 27, 2017) “Binary Ionization Technology (BIT) Solution”. EPA. Retrieved May 31, 2020.
53. ^ (Sept 25, 2019) “Accel TB Wipes”. EPA. Retrieved May 31, 2020.
54. ^ (Nov 8, 2017) “Accel (Concentrate) Disinfectant Cleaner”. EPA. Retrieved May 31, 2020.
55. ^ (Feb 21, 2020) “Peroxide Multi Surface Cleaner and Disinfectant”. EPA. Retrieved May 31, 2020.
56. ^ (Feb 27, 2019) “Clorox Pet Solutions Advanced Formula Disinfecting Stain & Odor Remover”. The Clorox Company. Retrieved May 31, 2020.
57. ^ (Aug 27, 2017) “Oxy-Team™ Disinfectant Cleaner”. EPA. Retrieved May 31, 2020.
58. ^ (Oct 5, 2017) “Peroxide Disinfectant And Glass Cleaner RTU”. EPA. Retrieved May 31, 2020.
59. ^ (Aug 15, 2019) “Oxy-1 Wipes (AHP)”. EPA. Retrieved May 31, 2020.
60. ^ (Feb 16, 2017) “Accel TB (AHP)”. EPA. Retrieved May 31, 2020.
61. ^ (March 4, 2019) “Oxy-1 RTU (AHP)”. EPA. Retrieved May 31, 2020.
62. ^ (Oct 5, 2017) “Suretouch (AHP)”. EPA. Retrieved May 31, 2020.
- Why Helicopter Money Is Inflationary - 2021-10-09
- How Interest Rates Affect Inflation - 2021-10-08
- How Long Has Inflation Existed? - 2021-10-01




