Yoguely is reader-supported. When you buy through links on our site, we may earn an affiliate commission. Learn more
How to Use Bleach Disinfectant

In today’s post I’m going to show you exactly how to use sodium hypochlorite, the active ingredient in bleach, the right way to clean and disinfect surfaces from 99.99% of microbes including COVID-19.
In fact, this is the same process I use to safely disinfect using sodium hypochlorite.
So if you would like to know “How to clean with sodium hypochlorite?”, you’ll love this guide.
Let’s jump into it!
How to Use Sodium Hypochlorite (Bleach) the Right Way
Sodium hypochlorite is a staple cleaning agent and the active ingredient in many household cleaners.
This alkaline cleaning agent is commonly referred to as bleach (paid link). It is diluted with water or other filler ingredients to a concentration of about 6.0% sodium hypochlorite (NAClO).

The hypochlorite (ClO−) releases chlorine (Cl). Which, is a toxic gas that attacks your respiratory system and is harmful for the environment.
When bleach reacts with colored organic compounds, it bleaches them. In other words, it removes their color and makes them white.[30]
Bleach should not be used on organic matter. Basically, when you react chlorine with organic matter it creates volatile organic contaminants (VOCs). VOCs are extremely harmful because they have been proven to be carcinogenic.
I dive deeper into this on my post on making distilled water at home.
Furthermore, bleach is neutralized when it comes in contact with dirt.[33] That is because dirt contains organic matter such as dead cells, hair, and bug carcasses that react with bleach.
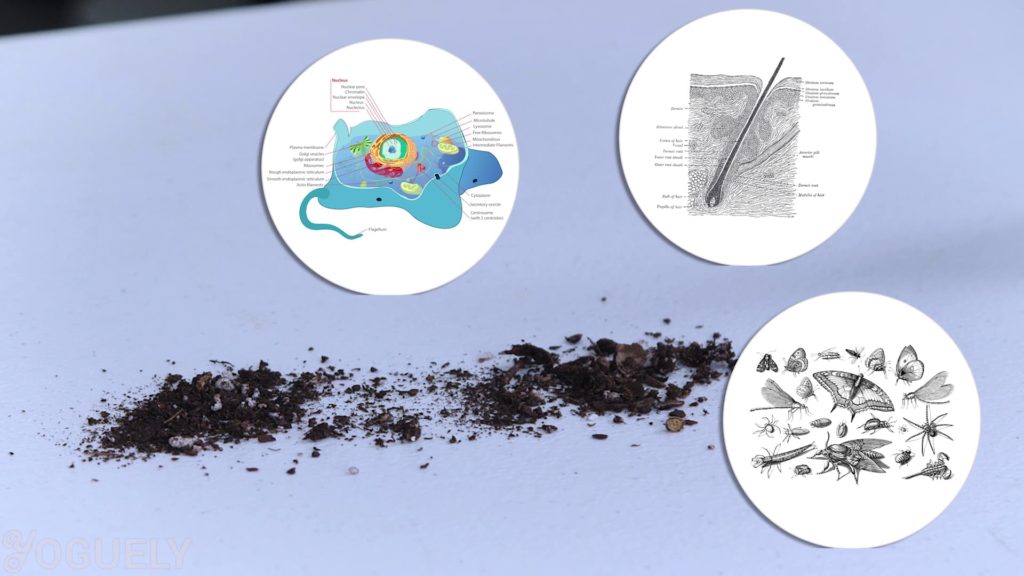
So before cleaning with bleach, you’ll need clean surfaces with detergent or soap and water to remove as much dirt as possible.
How Do You Make Sodium Hypochlorite Safer to Use at Home?
Even at 6% sodium hypochlorite concentration, bleach is very toxic and corrosive. Meaning it will eat through your skin, eyes, and lungs.
So you need to make bleach much safer to use while still being effective for bacteria and viruses like the current novel coronavirus.
To do so, simply dilute the bleach by mixing 1/3rd cup of bleach per gallon of water.[36] This will vary depending on the concentration of sodium hypochlorite in your bleach.
Do not mix bleach with any solvent other than water. Aside from being dangerous, mixing bleach with other chemicals can make it less effective. So you may end up wasting your time and money.
Sodium Hypochlorite Safety Measures
Here are some safety measures to take whenever working with bleach.
MSDS: Safety Data Sheet for Bleach at 5-10% sodium hypochlorite concentration.
Hazards: As far as hazards go, bleach is corrosive to metals and causes serious eye damage. Irritant to skin and lungs. It is very toxic to the environment, especially aquatic life with long lasting effects.

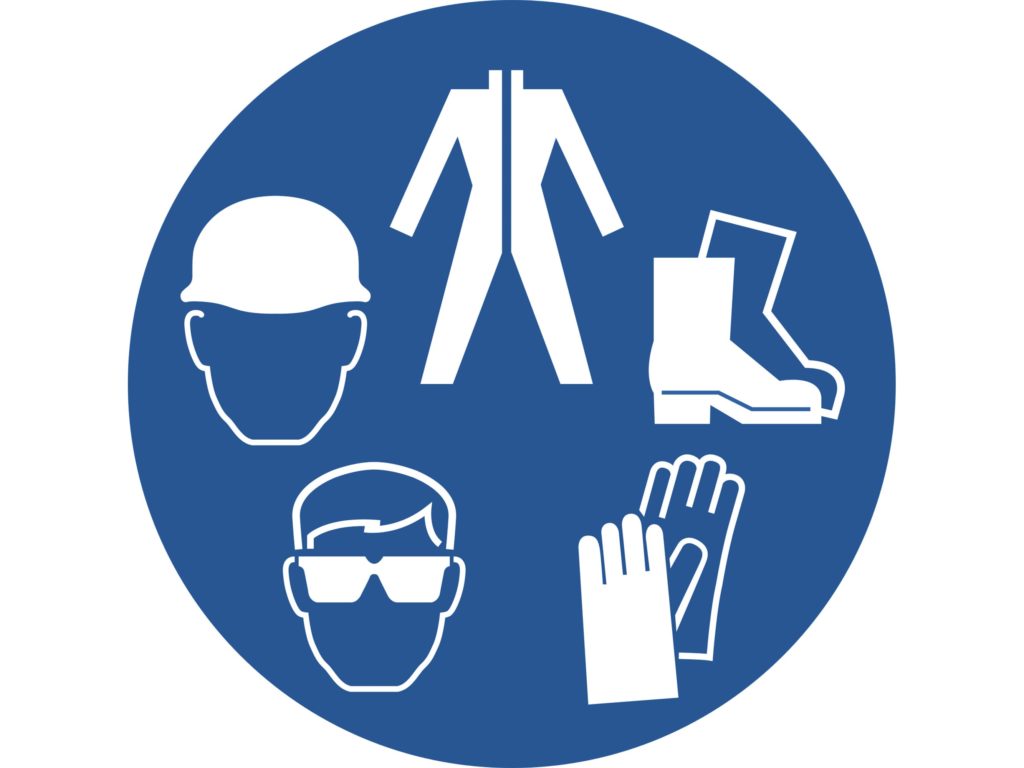
Personal Protective Equipment (PPE): Since bleach is toxic, you’ll need to wear some personal protective equipment (PPE) to stay safe.
In the first place, wear rubber gloves (paid link) or neoprene gloves (paid link) and protective clothing to cover all exposed skin.
Equally important, wear safety goggles (paid link) or full face shield. Lastly, wear respiratory protection (paid link) by the National Institute for Occupational Safety and Health (NIOSH) or the MSHA.[37]

Have an emergency eye wash and safety shower nearby.
And use an exhaust ventilation to keep airborne concentrations low.
What is Sodium Hypochlorite Good For?
Material it helps remove: Bleach is very effective in removing stains, damaging natural clothing materials, and weakening dyes.

Surfaces you can clean: Use bleach only on hard nonpurous surfaces.
Natural stones are porous. So do not use bleach on natural stones like marble and granite. You don’t want bleach to stay trapped in pores.

Bleach will corrode metals such as iron, stainless steel, aluminum, copper, and brass .[33] So avoid using bleach on metals unless you purposefully want to rust metals.
Is Sodium Hypochlorite a Disinfectant?
Bleach is effective in killing 99.99% of germs.
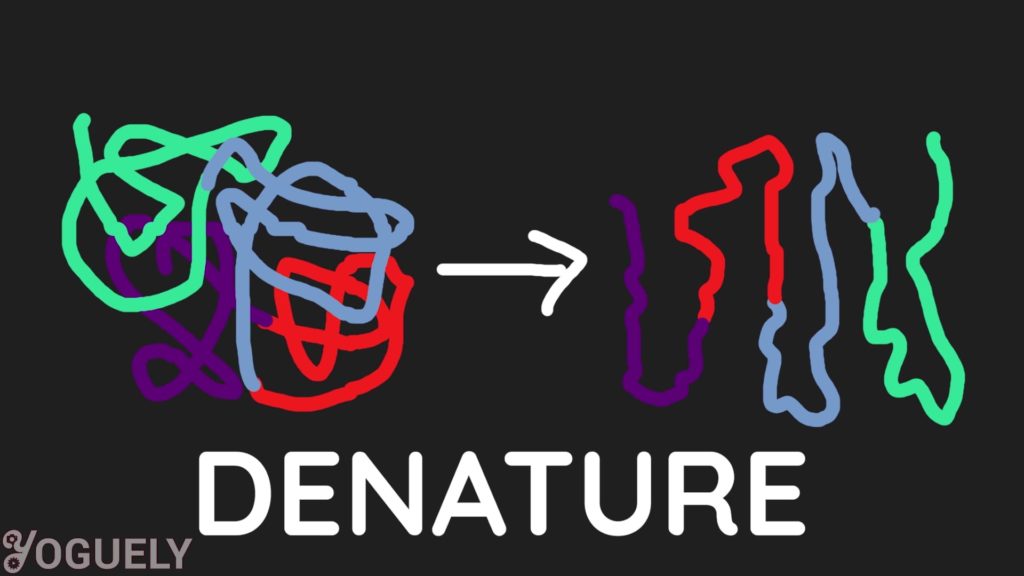
Bleach damages the protein structure of microbes which irreversibly denatures them or destroys microbes completely.[30] Which is why bleach is used to control bacteria, viruses, algae, and even mildew.
Can Sodium Hypochlorite Kill Coronavirus?
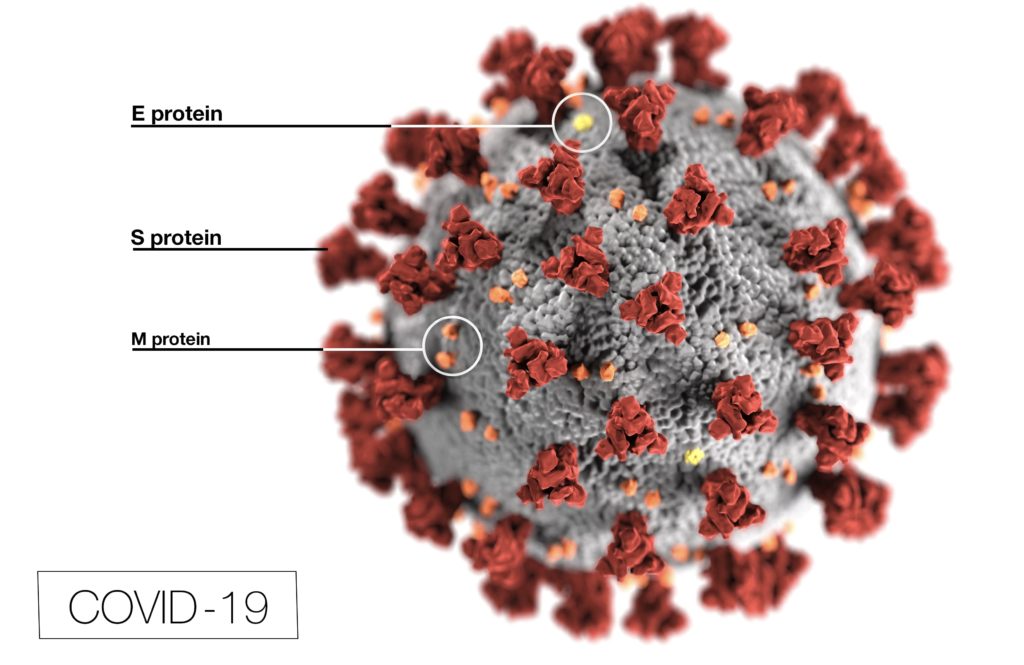
The Environmental Protection Agency (EPA) registers the active ingredient sodium hypochlorite as a disinfectant that is effective for SARS-CoV-2 (COVID-19).[32]
How Long Should Sodium Hypochlorite Sit on Surface to Disinfect?
According to the EPA, the contact time is the amount of time the surface should be visibly wet.[32]

Contact time: The surface you wish to disinfect will need enough contact time with bleach to kill the majority of germs.
So, the contact time will depend on the microbe you wish to destroy and the concentration of sodium hypochlorite used. For more info, check out the lists of EPA-Registered Disinfectants for common pathogens.[34]
I’ll be focusing on COVID-19, since it is one of the biggest threats to our well-being we face today.
The EPA recommends as much as 10 minutes of contact time with solutions of sodium hypochlorite to be effective against COVID-19.[32]
What is the Shelf Life of Sodium Hypochlorite?
Shelf life: Bleach has an definite shelf life.[33] Bleach breaks down into salt and water naturally. This process happens much faster when bleach is exposed to extreme temperatures, light, air, and contaminants. It is stable for at least 10 weeks, after which it’s antimicrobial strength decreases.[38]

When stored at room temperature, you may need to replace the bleach solution after 1 year.[39] Always mix solutions immediately before use.
How to Store Sodium Hypochlorite for Long Term?
Store bleach in a cool, dry place away from direct sunlight. And make sure it’s out of reach from children and animals.
Store bleach separately from other chemicals that could react adversely. Here are three mistakes you should avoid:
Do not in any way mix bleach with vinegar. It forms toxic chlorine gas which leads to pneumonitis and is fatal in large doses.

Never mix bleach with ammonia. Since, it forms toxic chloramine vapors which are a respiratory irritant.

Lastly, under no circumstances mix bleach with rubbing alcohol. That combination creates chloroform, a toxic anesthetic and sedative.

Conclusion
Alkaline cleaning agents are very dangerous to use. They are extremely toxic for you and can do irreversible damage to the environment.
I don’t like using them. So I avoid them at all costs.
There you have it folks. That’s my guide on how to use sodium hypochlorite the right way.
Leave your thoughts in the comment section below. Or join the discussion in the Yoguely Community Forum.
I’m Aida Yoguely. Thanks for learning with me today. And I’ll see you soon.
In my next post, learn about isopropyl alcohol and how to use it for disinfecting. To stay tuned, join our newsletter and get the latest content straight to your inbox.
Video
Be sure to subscribe and hit the notification bell to stay tuned for the latest videos.
References
11. ^ “Compound Summary: Sodium Hypochlorite”. National Library of Medicine. Retrieved June 1, 2020.
30. ^ (May 16, 2020). “Bleach”. Wikipedia. Retrieved May 26, 2020.
33. ^ (2013). “Green Cleaning, Santizing, and Disinfecting: A Curriculum for Early Care and Education”. EPA. Retrieved May 27, 2020.
32. ^ “List N: Disinfectants for Use Against SARS-CoV-2”. EPA. Retrieved May 27, 2020.
34. ^ (March 13, 2013). “Selected EPA-Registered Disinfectants”. EPA. Retrieved May 27, 2020.
36. ^ (April 2, 2020) “How to clean and disinfect”. CDC. Retrieved May 27, 2020.
37. ^ (January 1996) “NIOSH Guide to the Selection and Use of Particulate Respirators”. CDC. Retrieved May 27, 2020.
38. ^ Johnson BR, Remeikis NA. Effective shelf-life of prepared sodium hypochlorite solution. J Endod. 1993;19(1):40‐43. doi:10.1016/S0099-2399(06)81040-X
39. ^ (October 2010) “Shelf Life”. Clorox. Retrieved May 28, 2020.
- Why Helicopter Money Is Inflationary - 2021-10-09
- How Interest Rates Affect Inflation - 2021-10-08
- How Long Has Inflation Existed? - 2021-10-01




