Yoguely is reader-supported. When you buy through links on our site, we may earn an affiliate commission. Learn more
How to Use Isopropyl Alcohol Disinfectant (70% vs 91% vs 99%)

In today’s post I’m going to show you the exactly how to use isopropyl alcohol (IPA) the right way to disinfect surfaces from 99.99% of microbes including COVID-19.
In fact, this is the same process I use to safely disinfect using isopropyl alcohol.
So if you would like to know “How to disinfect with isopropyl alcohol?”, you’ll love this guide.
Let’s jump into it!
How to Use Isopropyl Alcohol the Right way
Isopropyl alcohol (IPA), also known as 2-propanol, is a staple cleaning agent. It is the active ingredient in many household cleaners.
It comes diluted with purified water or in sanitizer solutions. Typically, to a concentration of 70% to 99% concentration.

Sanitizers (paid link) are commonly made with purified water, glycerine, carborner thickening agent, and fragrance. Some hand sanitizers (paid link) also have added vitamin E, and aloe vera.

Alcohol-based sanitizers can inactivate microbes. But a common mistake is relying only on alcohol-based sanitizers to disinfect.
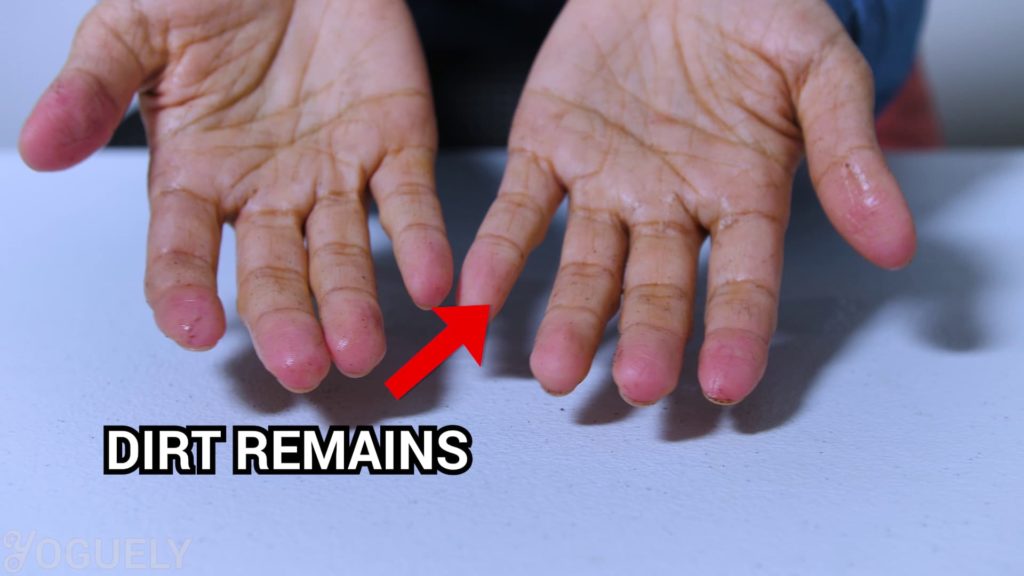
If you can, wash off the dirt and grease first.
Use soap and water for your hands or detergent for the surface you are cleaning. That way you reduce the number of microbes. Plus remove certain ones that alcohol cannot.[17]
Then apply the alcohol-based sanitizer afterwards to kill any remaining germs.

What is Isopropyl Alcohol Good For?
Material it helps remove: In terms of materials, isopropyl alcohol (paid link) is very effective at dissolving oils.
Surfaces you can clean: Isopropyl alcohol evaporates really quickly, eliminating almost all traces of oil. Given that fact, it is commonly used for metal, plastic, and glass materials.

For instance, you can use it for cleaning eyeglasses. It is safe to use for electronics. Such as: electrical contacts, audio or video tape heads, and optical disc lenses like DVDs.
It is also useful for removing thermal paste from heatsinks on CPU’s. Which is something I do once a year on my desktop PC build.

Is 70% Isopropyl Alcohol a Disinfectant?
70% Isopropyl alcohol is effective in killing 99.99% of germs.
Specifically, isopropyl alcohol diluted with 30% to 10% purified water is a rapid acting bactericidal. Meaning it’s capable of killing bacteria. It is also tuberculocidal, fungicidal, and virucidal.
Unfortunately though, they do not destroy bacterial spores.[2] Which explains why solutions of alcohol are not approved by the Food and Drug Administration (FDA) as a high-level disinfectant.[2]
How does alcohol kill germs?
Alcohol is an antimicrobial solution that works by denaturing the proteins of germs.[2] This in a way, is similar to how soap works. Alcohol unfolds proteins in living cells, which inactivates cell activity or kills the cell completely. This process is called denaturation.[3]
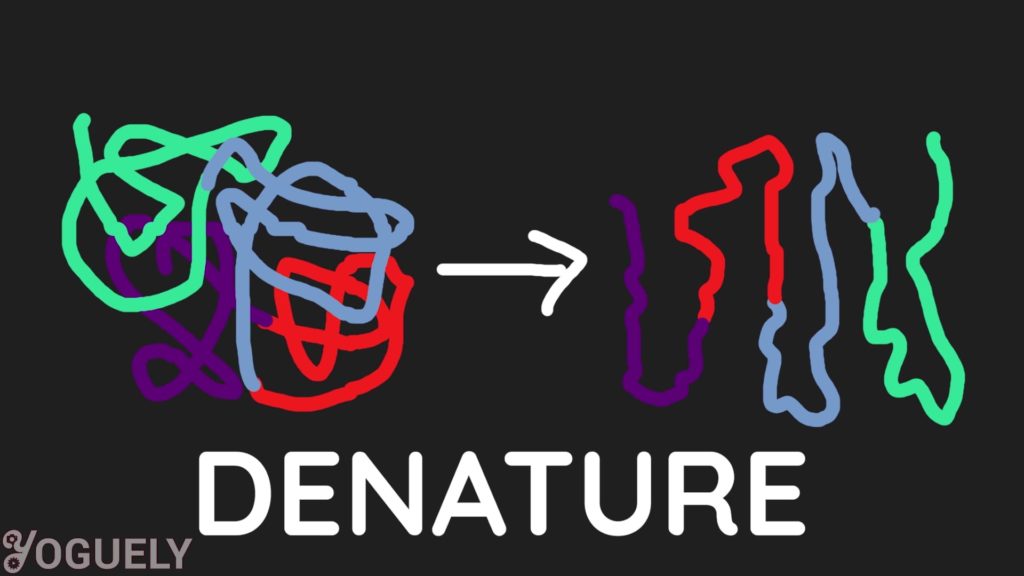
Mixtures of alcohol and purified water are more capable of killing bacteria than absolute alcohol. This is because proteins are denatured more rapidly when there is water present.[4],[5]
I’ll explain this is more detail later. For now you must be wondering:
Can 70% Isopropyl Alcohol Kill Coronavirus?
Isopropyl alcohol is fully active against lipid viruses.[7] Those are viruses who’s cell membrane are made of a lipid bilayer. In turns out, coronaviruses have a lipid bilayer. Therefore, isopropyl alcohol is very effective in inactivating COVID-19.
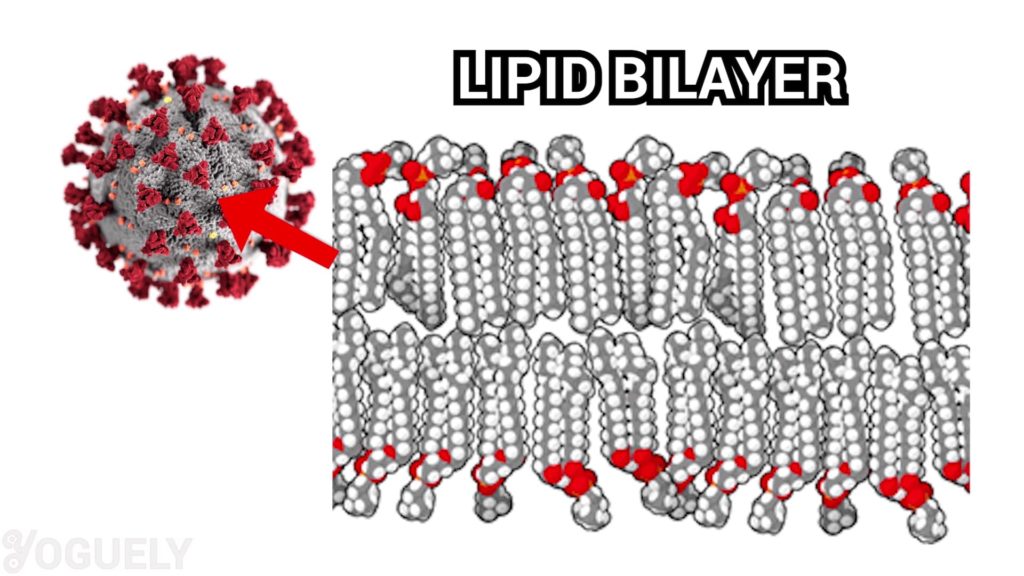
For this reason, the Environmental Protection Agency (EPA) registers the active ingredient isopropyl alcohol as an effective disinfectant for SARS-CoV-2 (COVID-19).[32] Specifically, the CDC also recommends using at least 70% alcohol to disinfect at home.[8],[33]
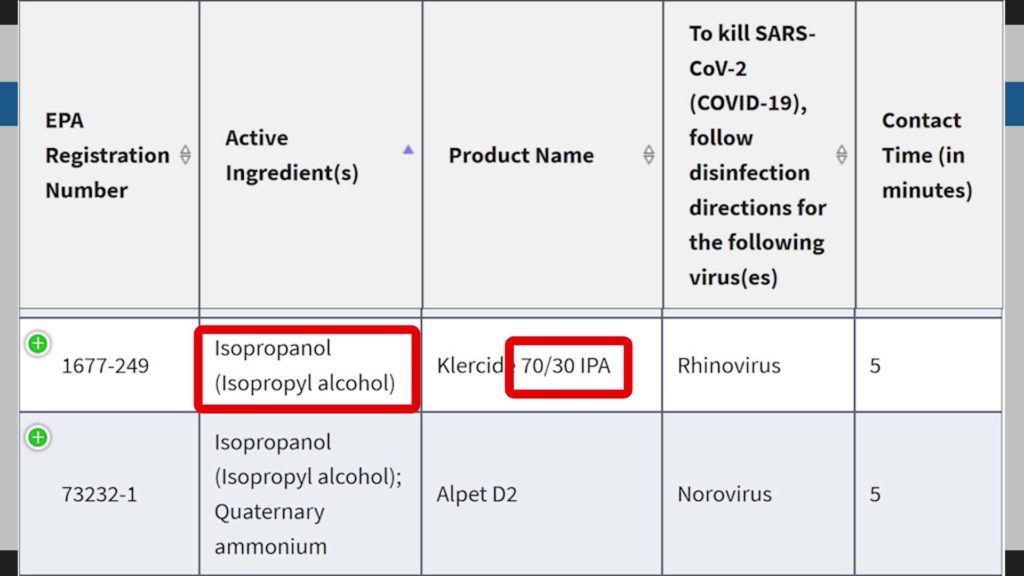
Other useful disinfectants that can be used against coronavirus are sodium hypochlorite (bleach), or hydrogen peroxide.
How Long Should 70% Isopropyl Alcohol Sit on Surface to Disinfect?
According to the EPA, the contact time is the amount of time the surface should be visibly wet.[32]
Contact time: The contact time you need may vary depending on the the microbe you wish to destroy. It also depends on the concentration of isopropyl alcohol used. For more info, check out the lists of EPA-Registered Disinfectants for common pathogens.[34]
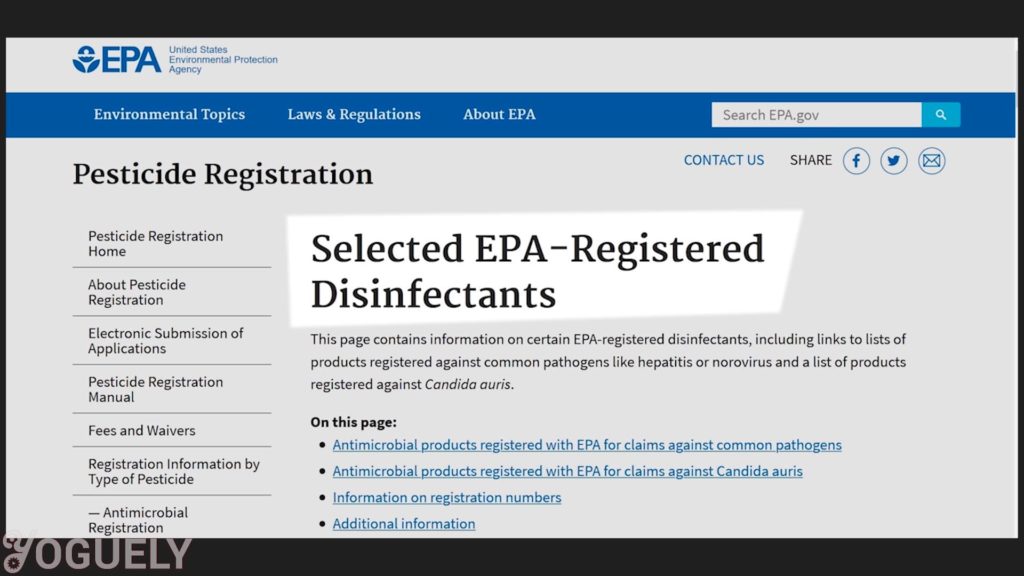
I’ll be focusing on COVID-19, since it is one of the biggest threats to our well-being we face today.
The EPA recommends as much as 5 minutes of contact time with solutions of 70% isopropyl alcohol and 30% water to be effective against COVID-19.[32]
But hold on,
How Long Does It Take for 70% Isopropyl Alcohol to Evaporate?
Alcohols evaporate VERY rapidly. How fast, will depend on the air exchange rate, and the temperature.
Alcohol’s fast dry times makes it difficult to expose a surface to extended contact time.[16] Unless, you immerse the surface completely under alcohol for the entire 5 minutes.
You could also experiment by drenching the surface in a thick layer of the liquid. Then measuring how long it takes to dry.
70% vs 91% vs 99% Isopropyl Alcohol (The Difference)
You’ve probably seen 3 concentrations of diluted isopropyl alcohol being sold. The difference is the ratio of isopropyl alcohol to purified water:
- 70% isopropyl alcohol and 30% purified water
- 91% isopropyl alcohol and 9% purified water
- 99% isopropyl alcohol and 1% purified water

Which is better for disinfecting 70%, 91%, or 99% isopropyl alcohol?
Here are 3 reasons why 70% isopropyl alcohol is, in most cases, the optimal concentration you will ever need at home.
- First, it is longer lasting.
- Second, it is more effective as a disinfectant.
- Third, it is safer to use and store.
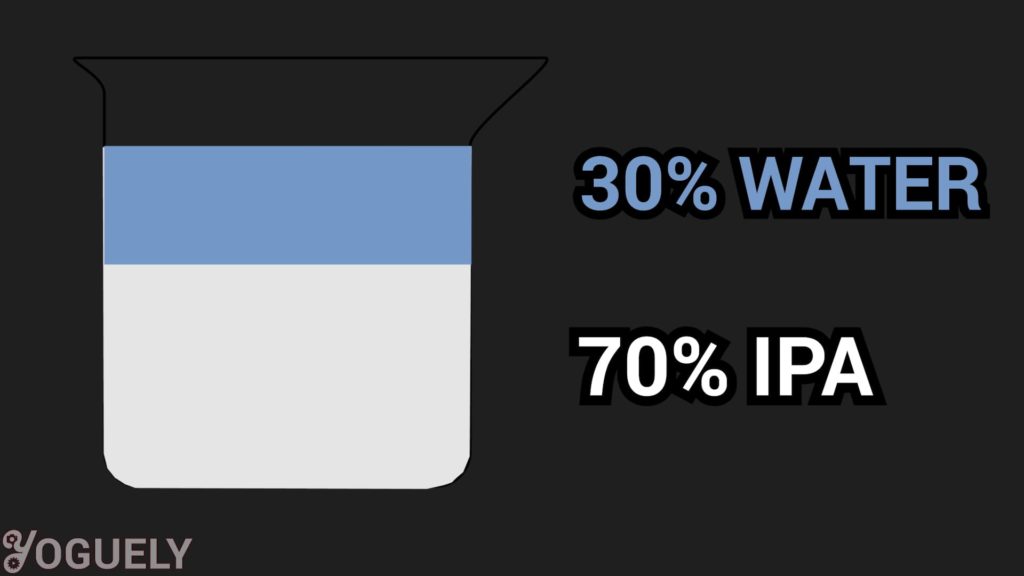
It Is Longer Lasting.
Alcohol evaporates very rapidly, much faster than water. Mixing alcohol with water actually slows down the evaporation rate. So every time you open and close a container of alcohol, less of it will be used up.
There is another bigger advantage though. By evaporating much slowly, 70% isopropyl alcohol sits on surfaces for longer. Which, increases the contact time, and hence its effectiveness.
It Is More Effective as a Disinfectant.
Another key point, we are looking for the best bactericidal. Like I mentioned earlier, alcohol and water is more effective as a bactericidal than alcohol alone.
Now here is the interesting fact. Research shows that as you dilute alcohol with purified water below 50% alcohol, the solution becomes less effective. The optimal concentration is actually between 60% and 90% alcohol.[2],[4],[6]
Particularly, 70% isopropyl alcohol lands within the optimal concentration range.
It Is Safer to Use and Store.
Isopropyl alcohol is highly flammable. The higher the concentration on alcohol, the more dangerous it is as a flammable solution. So you don’t want to be keeping high concentrations of it lying around.
For your safety at home, use the least concentration of isopropyl alcohol that is still effective against the microbe you want to eradicate.
70% isopropyl alcohol is the most diluted solution that still lies within the optimal concentration range recommended by the CDC.
For these 3 reasons: stability, effectiveness, and safety. And because of my experience in engineering applications. I go with 70% isopropyl alcohol whenever disinfecting at home.
Isopropyl Alcohol Safety Measures
Here are some safety measure to take whenever working with isopropyl alcohol.
MSDS: Safety Data Sheet for 70% Isopropyl Alcohol
Hazards: As far as hazards go, isopropyl alcohol is highly flammable. It is also an irritant so keep it away from your eyes and skin.

Another key point, alcohol evaporates rapidly. And if you breath it, it can make you feel drowsy or dizzy.
Personal Protective Equipment (PPE): To prevent those hazards when handling isopropyl alcohol, you should wear personal protective equipment (PPE).
First off, wear protective clothing and gloves (paid link) to cover your skin. Equally important, wear chemical safety goggles (paid link) that meets the OSHA’s protection regulations.[18],[19]
In like manner, wear respiratory protection (paid link) by the NIOSH or the MSHA.[10]

Have an emergency eye wash and safety shower nearby.
Also, use an exhaust ventilation to keep airborne concentrations low. Remove all possible sources of ignition. Likewise, all your electrical equipment should be explosion-proof.
Key point often overlooked, take precautionary measures against static discharges.

If you are using alcohol on your hands, make sure your hands are completely dry. So that all the alcohol has evaporated before you touch anything. Any small static charge could spark a fire, and cause serious burns.
Shelf Life of Isopropyl Alcohol
Shelf Life: A 70% isopropyl alcohol product will have a EXP expiration date on printed on it which is about 3 years from the date you purchase it. After 3 years, the product might still be usable as long as the cap was sealed well after every use.
Here is why:
If you don’t seal the cap well, alcohol will begin to evaporate much faster than water.
Specifically, the boiling point of isopropyl alcohol is 82.6 °C (180.7 °F; 355.8 K). Which, is much lower than the boiling point of water 100 °C (212 °F; 373.15 K).
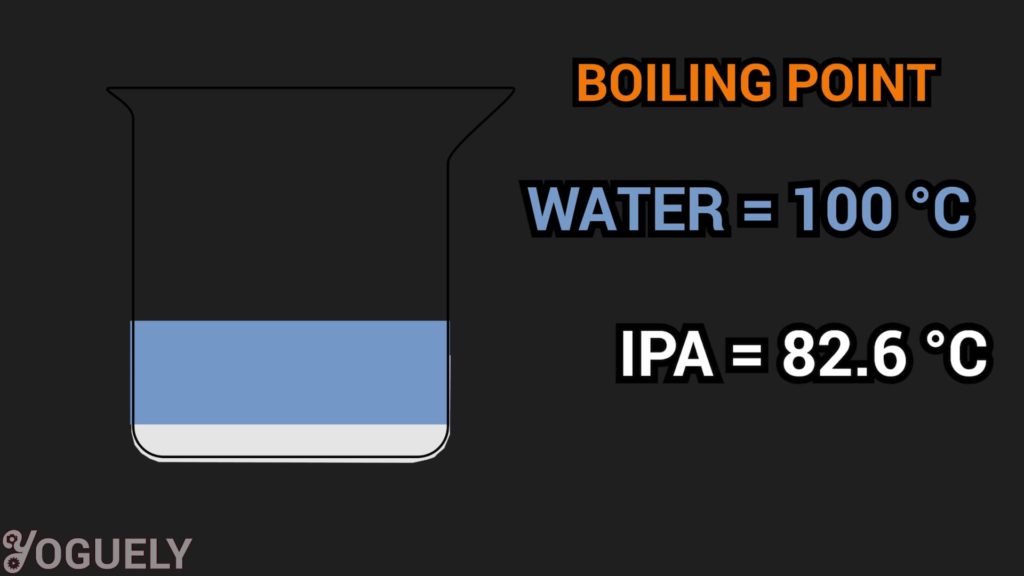
Over time, the concentration of alcohol will be much less, and the solution that remains will only contain water.
Besides it’s effectiveness, there is another thing you need to consider. That is, the danger of using isopropyl alcohol that has been exposed to air and then stored for a long time.
Isopropyl alcohol is a peroxide forming chemical. So it will react with air or oxygen to form unstable peroxides. These peroxides could accumulate at extremely dangerous concentrations. If that happens, it can cause an explosion.[11],[12],[13]

In fact, there already has been explosions in lab experiments due to peroxide formation from isopropyl alcohol.[14],[15]
So it may be a good idea to simply use up the entire bottle before its expiration date to avoid having a bad day.
How to Store Isopropyl Alcohol for Long Term
Alcohols should be stored in a fire resistant or preferably a fireproof storage.[9] That storage should be located in a cool, well-ventilated area, and away from heat sources that may cause a fire.
Alcohol is probably the most common flammable chemical I’ve worked with at NASA. I recall always storing it in a fire rated chemical storage cabinets. Kind of like this flammable’s mini safety storage cabinet (paid link), but way bigger.

Store alcohol separately from other chemicals that could react adversely. Especially since it is flammable.
So you’ll want to keep it away from strong oxidants like hydrogen peroxide.
Remember to never mix alcohol with bleach. Because it forms chloroform, a toxic anesthetic and sedative.

Conclusion
There you have it folks. That’s my guide on how to use isopropyl alcohol the right way.
Leave your thoughts in the comment section below. Or join the discussion in the Yoguely Community Forum.
I’m Aida Yoguely. Thanks for learning with me today. And I’ll see you soon.
To stay tuned, join our newsletter and get the latest content straight to your inbox.
Video
Be sure to subscribe and hit the notification bell to stay tuned for the latest videos.
References
1. ^ (May 28, 2020). “Isopropyl alcohol”. Wikipedia. Retrieved June 1, 2020.
2. ^ (Sept 18, 2018). “Guideline for Disinfection and Sterilization in Healthcare Facilities (2008)”. CDC. Retrieved May 30, 2020.
3. ^ (May 8, 2020). “Denaturation (biochemistry)”. Wikipedia. Retrieved May 23, 2020.
4.^ Ali Y, Dolan MJ, Fendler EJ, Larson EL. Alcohols. In: Block SS, ed. Disinfection, sterilization, and preservation. Philadelphia: Lippincott Williams & Wilkins, 2001:229-54.
5. ^ Morton HE. Alcohols. In: Block SS, ed. Disinfection, sterilization, and preservation. Philadelphia: Lea & Febiger, 1983:225-239.
6. ^ Morton HE. The relationship of concentration and germicidal efficiency of ethyl alcohol. Ann N.Y. Acad. Sci. 1950;53:191-96.
7. ^ Klein M, DeForest A. The inactivation of viruses by germicides. Chem. Specialists Manuf. Assoc. Proc. 1963;49:116-8.
8. ^ (April 2, 2020). “Cleaning And Disinfecting Your Home”. CDC. Retrieved June 1, 2020.
9. ^ “Laboratory Chemical Safety Summary (LCSS) Isopropyl alcohol”. National Library of Medicine. Retrieved June 1, 2020.
10. ^ (January 1996). “NIOSH Guide to the Selection and Use of Particulate Respirators”. CDC. Retrieved May 27, 2020.
11. ^ “Compound Summary: Isopropyl alcohol”. National Library of Medicine. Retrieved June 1, 2020.
12. ^ “Peroxide Formation in Chemicals”. UC Davis. Retrieved June 1, 2020.
13. ^ Clark, D. E. (2001). Peroxides and peroxide-forming compounds. Chemical Health and Safety, 8(5), 12-22.
14. ^ Mirafzal, G. A., & Baumgarten, H. E. (1988). Control of peroxidizable compounds: An addendum. Journal of Chemical Education, 65(9), A226.
15. ^ (Aug 1, 2016). “Chemical safety: peroxide formation in 2-propanol“. Chemical & Engineering News. Retrieved June 1, 2020.
16. ^ Venkataram, M. (2012). Textbook on Cutaneous and Aesthetic Surgery. JP Medical Ltd.
17. ^ (March 3, 2020). “Show Me the Science – When & How to Use Hand Sanitizer in Community Settings“. CDC. Retrieved June 2, 2020.
18. ^ “Eye and Face protection“. United States Department of Labor Occupational Safety and Health Administration. Retrieved June 6, 2020.
19. ^ “Selecting PPE for the Workpace“. United States Department of Labor Occupational Safety and Health Administration. Retrieved June 6, 2020.
32. ^ “List N: Disinfectants for Use Against SARS-CoV-2”. EPA. Retrieved May 27, 2020.
33. ^ (July 10, 2020). “Cleaning And Disinfection for Households”. CDC. Retrieved September 25, 2020.
- Why Helicopter Money Is Inflationary - 2021-10-09
- How Interest Rates Affect Inflation - 2021-10-08
- How Long Has Inflation Existed? - 2021-10-01




